Traditional oxidizing biocides, such as sodium hypochlorite (bleach) and gaseous chlorine, are commonly used in bulk water sanitization applications. However, these chemistries can cause significant operational challenges. You can potentially avoid these problems and significantly reduce your operating costs by switching to a novel chlorine stabilizer chemistry.
Traditional oxidizing biocides have very limited influence on biofilm. Biofilm forms on wet surfaces, piping in water distribution systems, cooling tower fill and heat exchangers. It forms when microorganisms in the water begin to adhere to surfaces and multiply. As the biofilm grows, it entrains nutrients and debris in the water, thereby forming an ever-thicker layer that becomes increasingly challenging to control. Mechanical cleaning or penetrating/dispersing chemistries may be required to remove deposits and biofilm.
A water contact surface that has slime dripping from it or that feels slippery—clearly indicates the presence of a biofilm and that the microbiological program is out of control. However, the impact of biofilm occurs long before it becomes visible and tactile by physical inspection. Biofilm acts as an excellent insulator because it contains a high percentage of trapped water with a high heat capacity. In a heat exchanger, as few as 20 microns of biofilm (1000 microns = 0.04 inches) can impede heat transfer by up to 7%.[1] In a cooling tower fill, biofilm reduces the ability of water to evaporate effectively, which is the mechanism for ultimately rejecting heat from the process. The net effect is an inability to effectively and efficiently cool process equipment such as vacuum pumps, surface condensers, turbine condensers or other utility heat exchangers.
A typical decision resulting from a lack of a holistic approach is the selection of bleach as the biocide of choice. Bleach is inexpensive, acts quickly, and kills bacteria in the bulk circulating water thus enabling plant operators to achieve bulk water bacterial count targets. However, three major problems occur with bleach, and they far outweigh its low cost.
The primary problem with bleach is that it is effective only at killing bacteria that are freely distributed in the recirculating water; it does little to control biofilm unless fed in excess, which creates other plant-wide issues. Once a biofilm forms, bleach is unable to penetrate and disperse it.[2]
Two other problems with bleach are that it is highly corrosive and that it is not persistent. Bleach is highly corrosive because of its strong and indiscriminate oxidizing capacity. This results in accelerated corrosion of metal components within the plant’s cooling system, including heat exchangers and piping. Significant cost, including reduced expected lifespan of equipment and increased maintenance, can be attributed to incorrect bleach use. In addition, sodium hypochlorite oxidizes metals. Oxidation of metals can be beneficial if clarification or filtration removes the oxidized metals. However, if not removed, the oxidized metals can accumulate on metal surfaces resulting in differential aeration cells that advance corrosion. Additionally, as a nutrient for microorganisms, oxidized metals can contribute to microbiologically influenced corrosion. Iron and manganese are the primary metals implicated in these corrosion mechanisms.
Bleach is not persistent in large circulating systems. An excess may be required in some parts of the system to maintain an adequate residual value in other parts of the system. As a strong oxidizer, bleach reacts quickly with organics and metals in the system, thereby competing with its ability to kill bacteria. This leads to variable residuals throughout the plant.

Figure 1 – OnGuard™ 3B analyzer.
Unique Chemistry Solution
Solenis has developed a novel chlorine stabilization chemistry that offers exceptional improvements in biofilm control. This patented chemistry is used in combination with sodium hypochlorite to produce an in-situ stabilized active chlorine solution. The resulting solution effectively controls both planktonic bacteria and biofilm in influent water, process water and cooling tower systems. The in-situ stabilized active chlorine solution is safe and easy to use and does not cause any of the adverse side effects associated with using strong oxidizing biocides. And, in many cases, the use of the chemistry has enabled customers to significantly reduce their operating costs.
Unique Equipment Solution
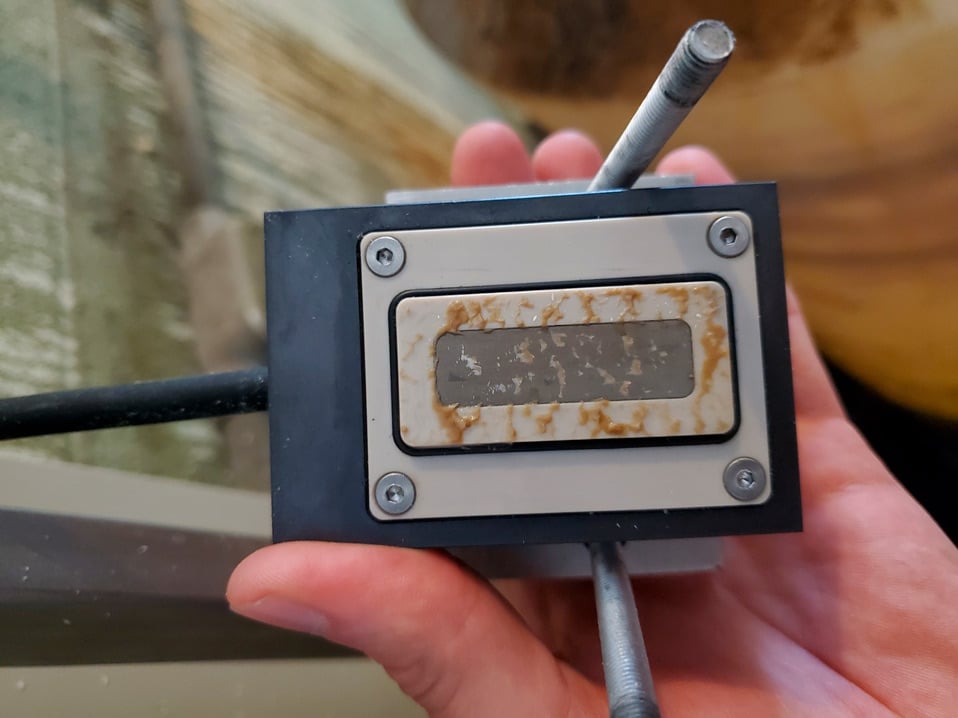
Figure 2 – OnGuard™ 3B analyzer sensor target showing biofilm fouling.
In 2015, Solenis developed a novel biofilm monitoring device that accurately measures biofilm growth. The device, which is marketed as the OnGuard™ 3B analyzer (see Figure 1), mimics critical heat exchanger conditions in real time by duplicating the shear stress on a surface while also simulating the local surface temperature. The exchanger fouling factor can be measured continuously and trended with integrated treatment program changes. Performance results can compare design and operating conditions of any heat exchanger of interest. While simulating heat transfer, foulant thickness determination is possible within an accuracy of +/- 5 microns (0.005 mm) using ultrasound. The device differentiates between biological, organic and scale deposits. Figure 2 shows the ultrasonic sensor target in a heavily fouled system. Effective use of this monitoring tool allows for optimization of chemistry applications and improves key performance indicators (KPIs). Biofilm avoidance can be targeted with a high degree of accuracy by analyzing trends and then adjusting microbicide feed. The result is optimal feed without having to overtreat to maintain target KPIs.
Unique Service Solutions
Solenis representatives have unique service capabilities while deploying this novel, enhanced chlorine stabilizer chemistry. Our digital platform allows our account management representative, as well as your plant representatives, 24/7, remote or direct access to program performance data. Additionally, the digital platform allows for notifications to be sent, as desired, to communicate any deviation from standard operation, allowing for quick response.
ClearPoint℠ Program
The unique solutions highlighted above are the foundation of Solenis’ ClearPoint℠ biofilm detection and control program. This novel program combines advanced chemistry, patented equipment and expert service to provide a comprehensive safeguard against microbiological activity and biofilm in industrial water systems.
Figure 3 shows a customer’s cooling tower distribution deck before and six weeks after treatment with the ClearPoint program.


Figure 3 – Cooling tower distribution deck before treatment and six weeks after treatment with the ClearPoint℠ program.
To learn more about these solutions, please visit our ClearPoint program webpage.
References
-
Knudsen, J.G. and Roy, B.V. (1982, September 6-10). Influence of fouling on heat transfer [Conference presentation]. International Heat Transfer Conference 7, Munich, Germany.
-
LeChevallier, M.W., Cawthon, C.D. and Lee, R.G. (1988). Factors promoting survival of bacteria in chlorinated water supplies. Applied and Environmental Microbiology, 54(3), 649–654.