Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to, Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), and Human Immunodeficiency Virus (HIV), the virus that causes AIDS. Workers exposed to bloodborne pathogens are at risk for serious or life-threatening illnesses.
Hepatitis: simply means ‘inflammation of the liver’. This inflammation can be caused by a group of viruses that specifically affect the liver. The most common types of hepatitis are hepatitis A, hepatitis B, and hepatitis C. The delivery of healthcare has the potential to transmit blood-borne hepatitis to both healthcare workers and patients. Outbreaks have occurred in outpatient settings, hemodialysis units, long-term care facilities, and hospitals, primarily as a result of unsafe injection practices; reuse of needles, finger stick devices, and syringes; and other lapses in infection control.
HIV, HBV and HCV are spread by contact with the blood and other potentially infectious materials (OPIM) of an infected person. The spread of these viruses from one person to another in healthcare settings is rare but can occur. This contact is primarily through contaminated needles, syringes, or other sharp instruments.
Medical experts emphasize that the careful practice of infection control procedures, including standard precautions (i.e., using protective practices and personal protective equipment to prevent transmission of these viral infections and other blood-borne infections), protects patients as well as healthcare providers from possible transmission in medical and dental settings.
All of the requirements of the Occupational Safety and Health Administration’s (OSHA)’s Bloodborne Pathogens standard can be found in the Code of Federal Regulations Title 29, Subtitle B, Chapter XVII, Part 1910, 1910.1030, at https://ecfr.federalregister.gov/current/title-29/subtitle-B/chapter-XVII/part-1910/section1910.1030
The standard’s requirements outline what employers must do to protect workers who are occupationally exposed to blood or other potentially infectious materials (OPIM), as defined in the standard. That is, the standard protects workers who can reasonably be anticipated to come into contact with blood or OPIM as a result of performing their job duties. This does not only apply to healthcare.
“A review of the initial intent of the Bloodborne Pathogens Standard that specifically deals with the cleaning of contaminated work surfaces, i.e., 1910.1030(d)(4)(ii)(A), reveals that OSHA intended to provide a performance-based provision that would allow for future development of “appropriate disinfectant” products. OSHA has reviewed the information on the disinfectants and has reconsidered its position on EPA-registered disinfectants that are labeled as effective against HBV and HIV. OSHA’s current stance is that EPA-registered disinfectants for HIV and HBV meet the requirement in the standard and are “appropriate” disinfectants to clean contaminated surfaces, provided such surfaces have not become contaminated with agent(s) or volumes of or concentrations of agent(s) for which higher level disinfection is recommended.
It is important to emphasize the EPA-approved label section titled “SPECIAL INSTRUCTIONS FOR CLEANING AND DECONTAMINATION AGAINST HIV-1 AND HBV ON SURFACES\OBJECTS SOILED WITH BLOOD\BODY FLUIDS.”
On the labels that OSHA has seen, these instructions require:
*Source: https://www.osha.gov/html/faq-bbp.html (last accessed September 22, 2020) With testing, manufacturers can indicate the minimum time required to inactive blood borne pathogens.
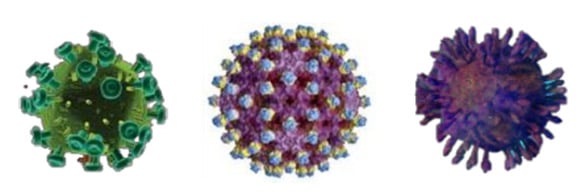
Bloodborne pathogens are enveloped viruses that are susceptible to the following Diversey disinfectants: